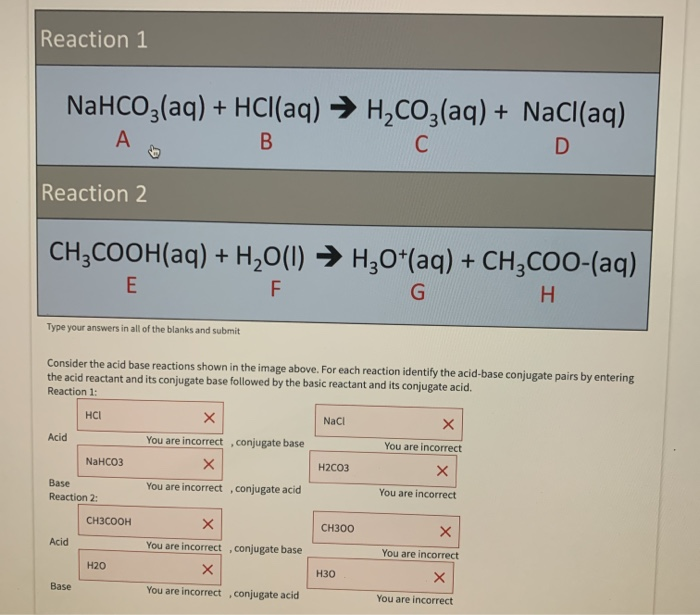
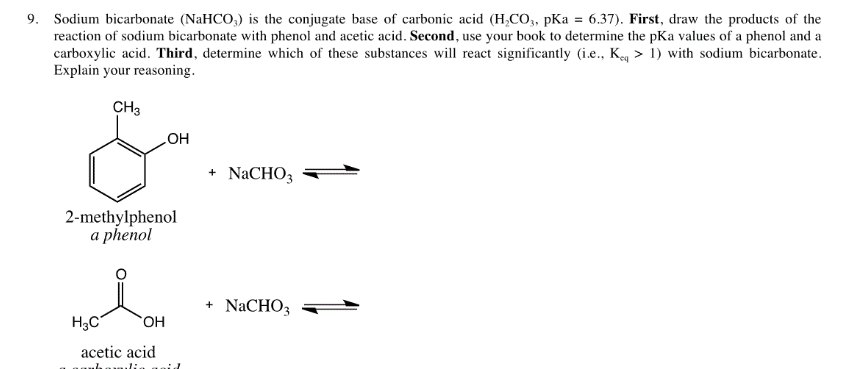
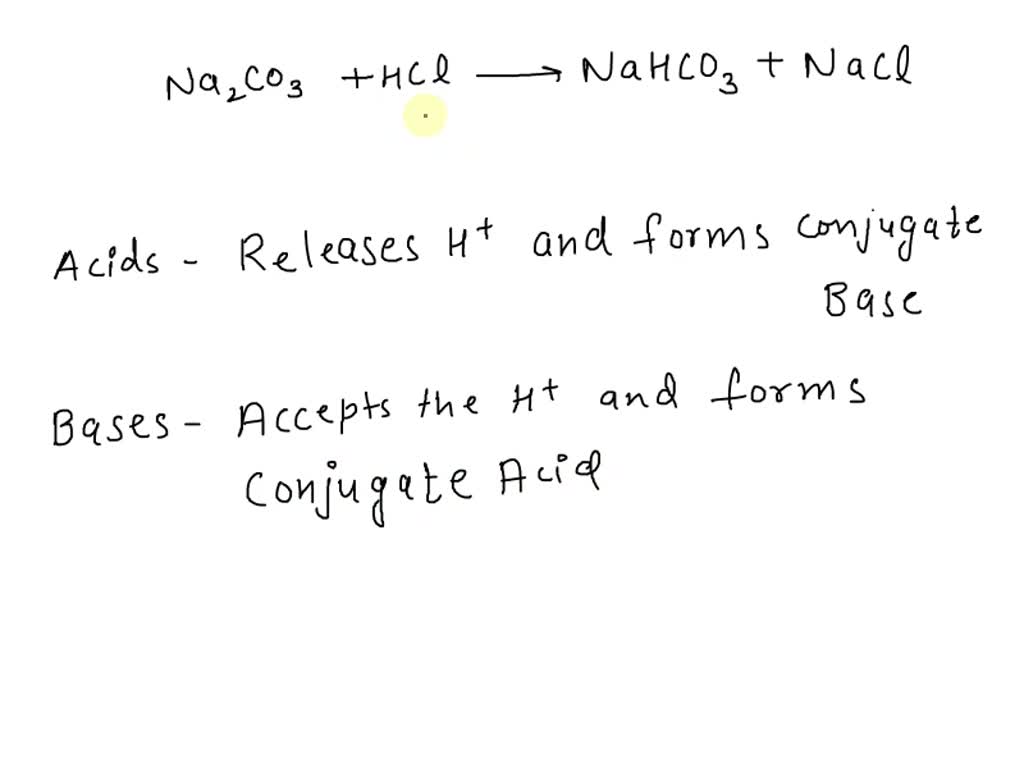
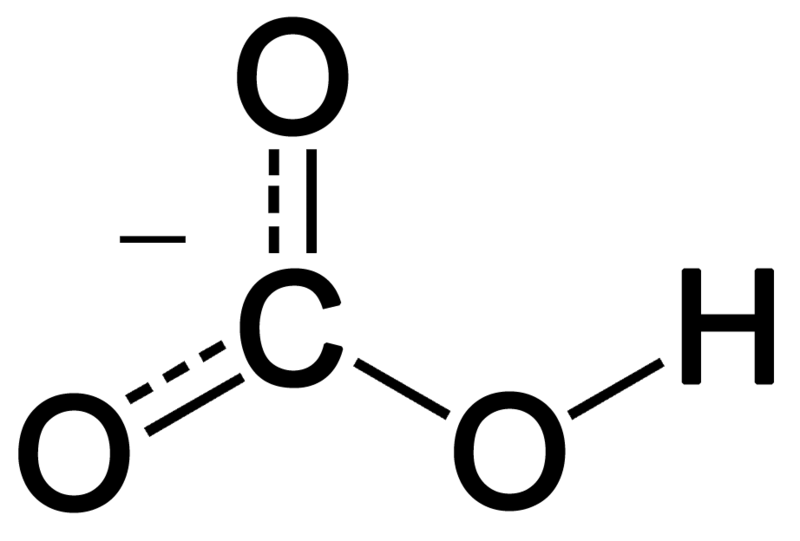
![SOLVED:A sodium hydrogen carbonate -sodium carbonate buffer is to be prepared with a pH of 9.40 . (a) What must the [HCO3^-] /[CO3^2-] ratio be? (b) How many moles of sodium hydrogen SOLVED:A sodium hydrogen carbonate -sodium carbonate buffer is to be prepared with a pH of 9.40 . (a) What must the [HCO3^-] /[CO3^2-] ratio be? (b) How many moles of sodium hydrogen](https://cdn.numerade.com/previews/ef6e1b45-03cf-464d-9e55-544d05108206_large.jpg)
SOLVED:A sodium hydrogen carbonate -sodium carbonate buffer is to be prepared with a pH of 9.40 . (a) What must the [HCO3^-] /[CO3^2-] ratio be? (b) How many moles of sodium hydrogen

Using aqueous hydrochloric acid, sodium bicarbonate, or sodium hydroxide solutions, devise a flow-chart separation scheme to separate the following two-component mixtures. Both substances are soluble | Homework.Study.com

How to Balance NaHCO3 + HCl = NaCl + CO2 + H2O (sodium bicarbonate plus hydrochloric acid) - YouTube