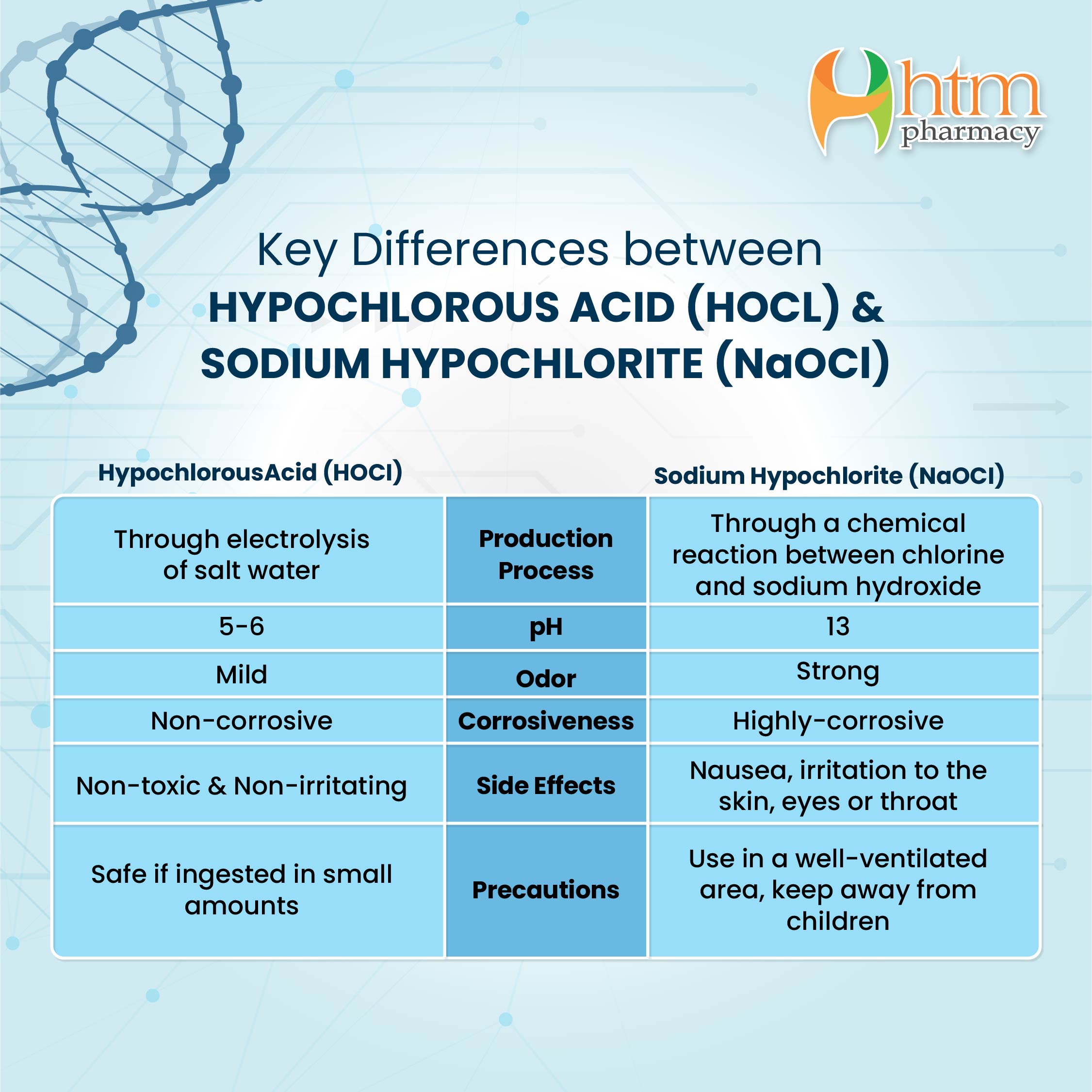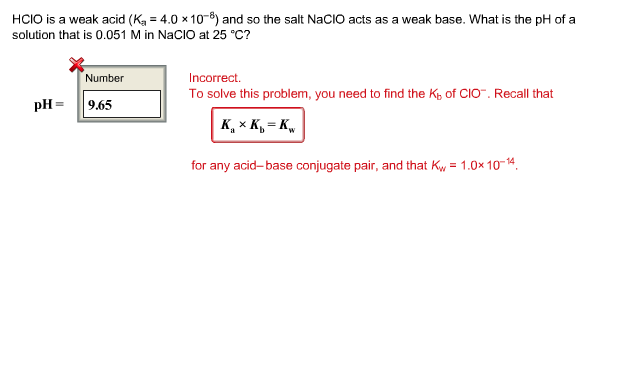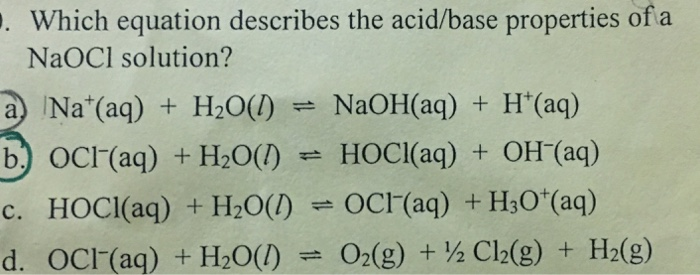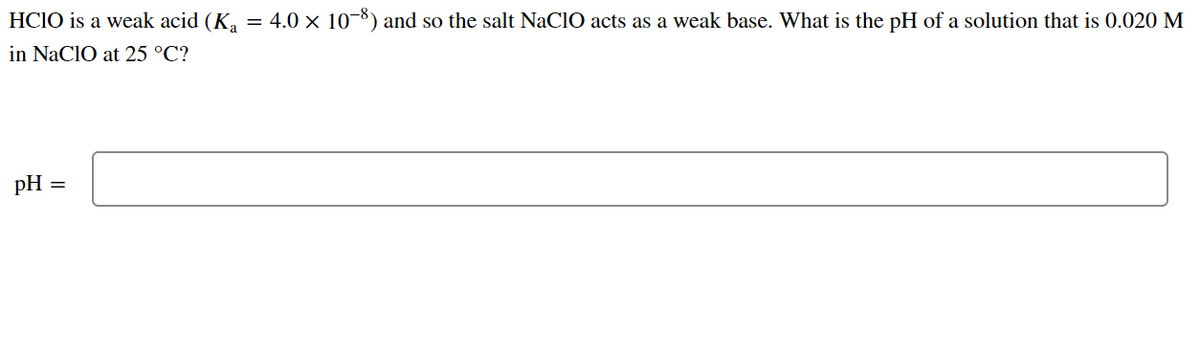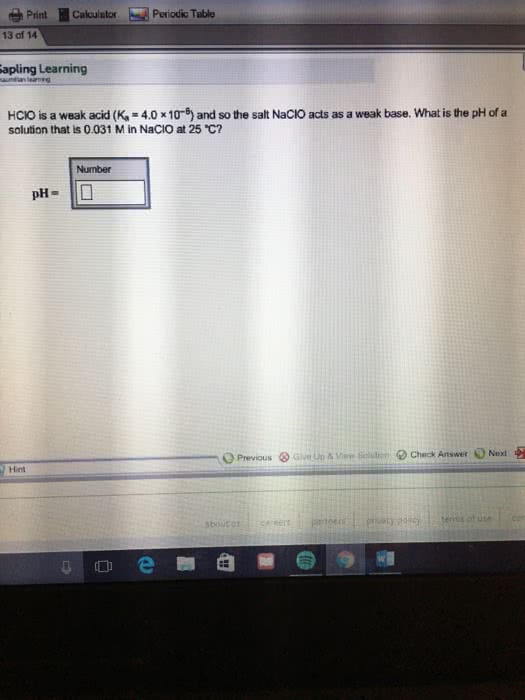
OneClass: HClO is a weak acid (Ka = 4x10^-8) and so the salt NaClO acts as a weak base. What is the p...

HClO is a weak acid (Ka = 4.0 x 10-8) and so the salt NaClO acts as a weak base. What is the pH of a solution that is 0.034 M in
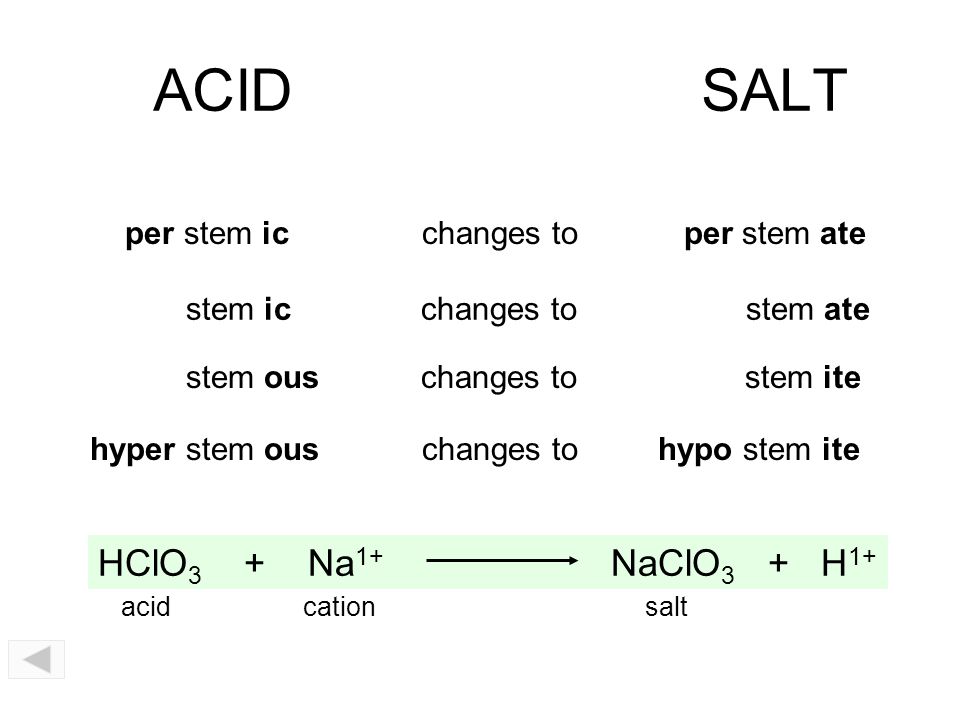
Acids, Bases, and Salts You should be able to Understand the acid-base theories of Arrhenius, Brønsted-Lowry, and Lewis. Identify strong acids and. - ppt download

A). Acid-base titration of raw MWCNTs I=0.01M NaClO 4 and T=20 ºC. The... | Download Scientific Diagram

Lab 24 - Hydrolysis A salt formed between a strong acid and a weak base is an acid salt. Ammonia is a weak base, and its salt with any strong acid gives. -
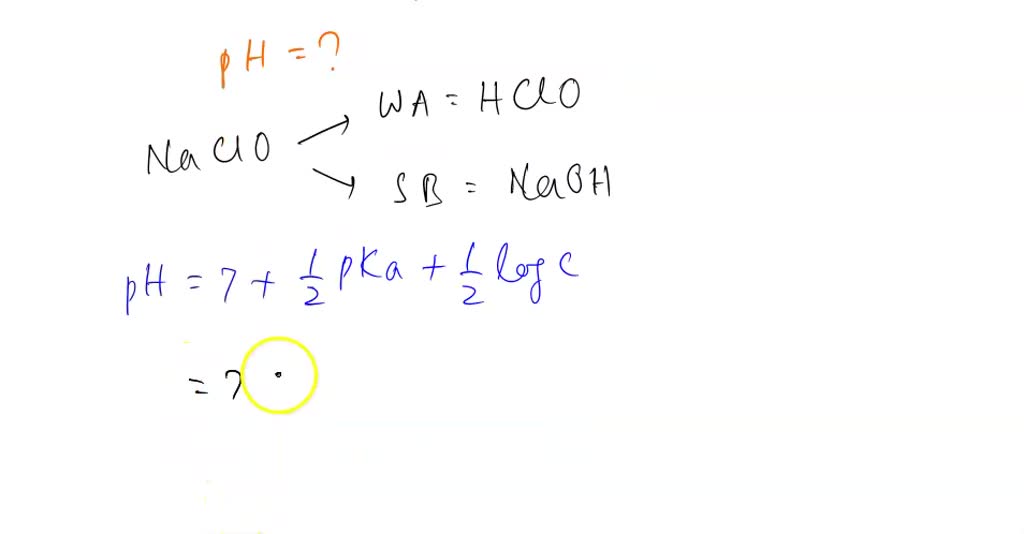
SOLVED: (A-D are completed, only E-L only need to be completed) Thank you! 1) Determine the pH of a 0.26 M solution of sodium hypochlorite (NaClO)? Ka for HClO = 3.5 x