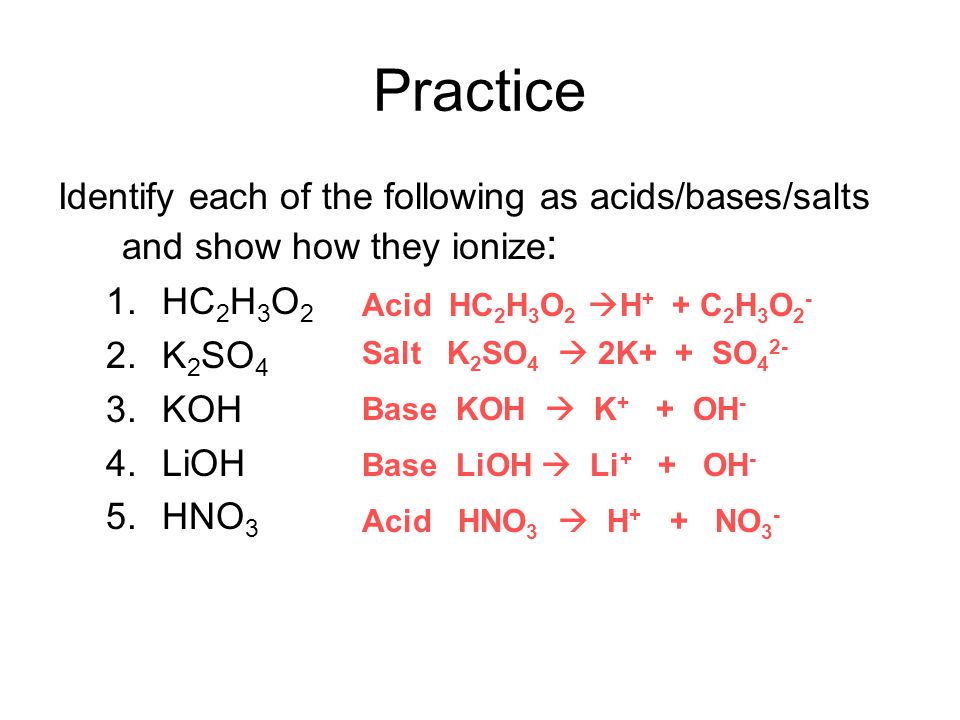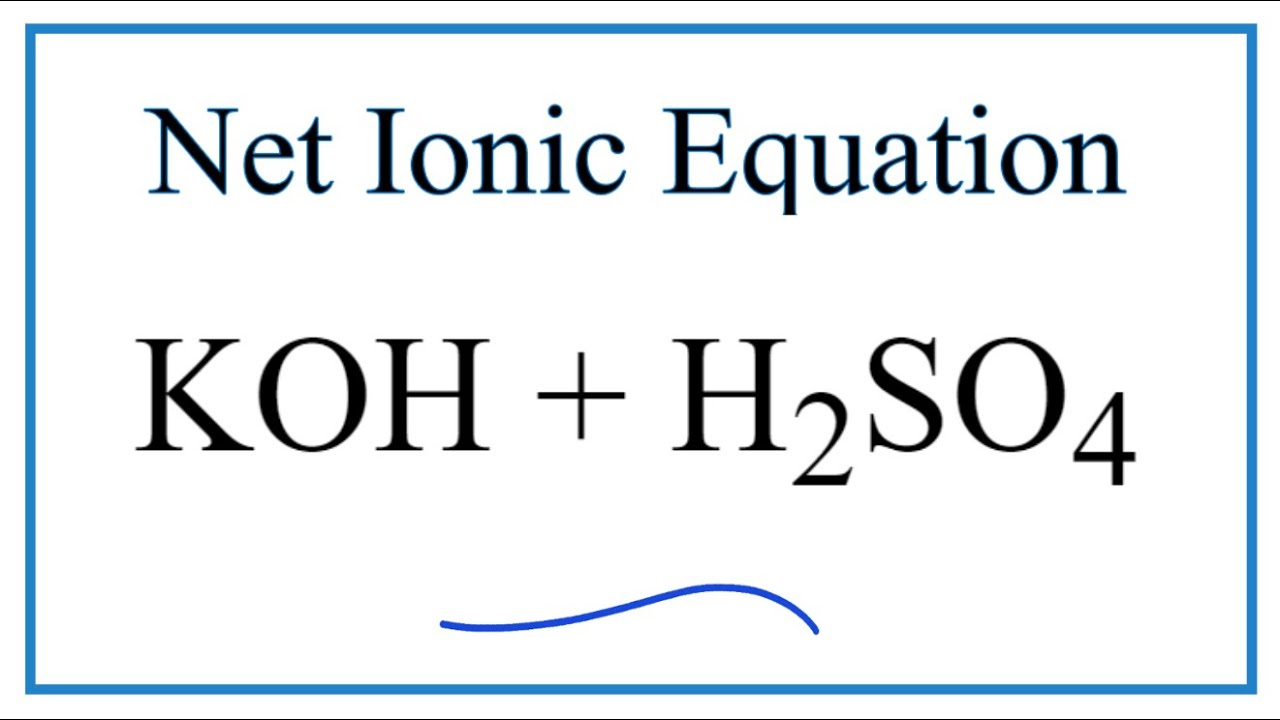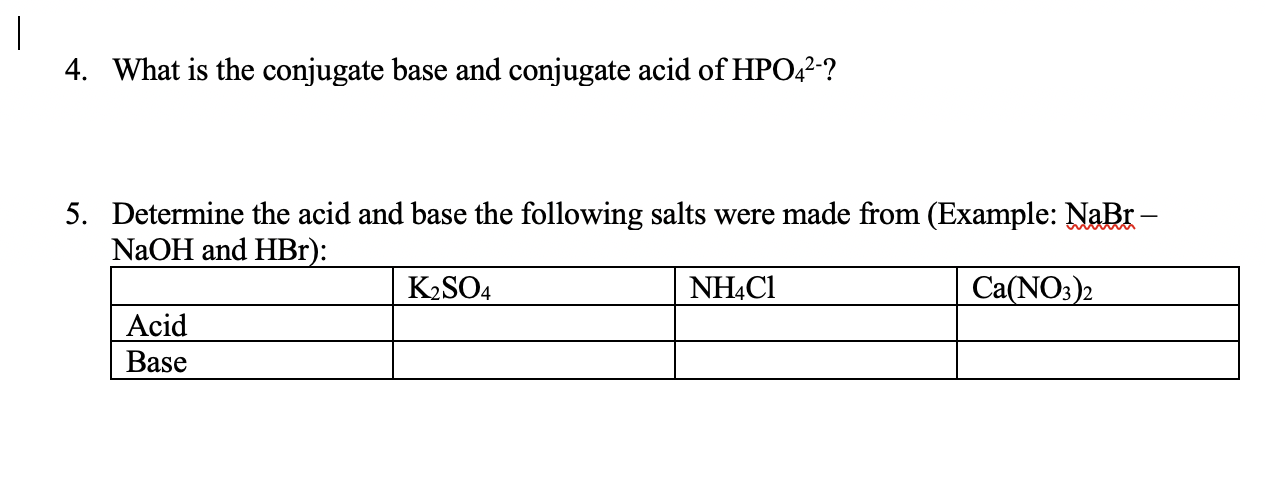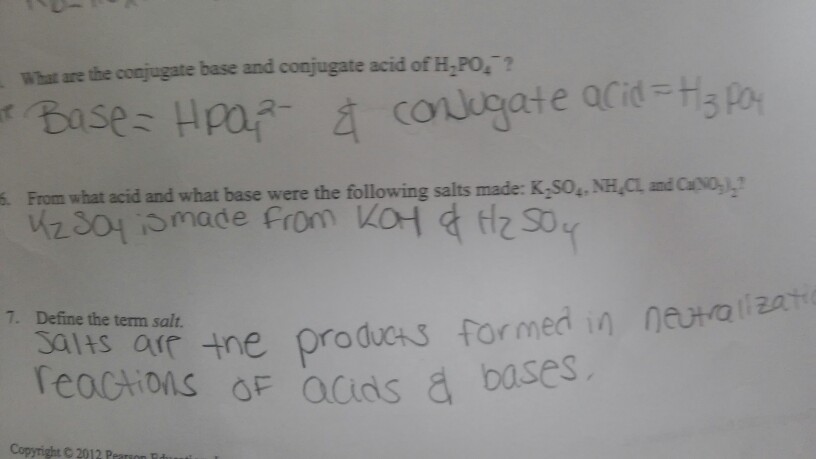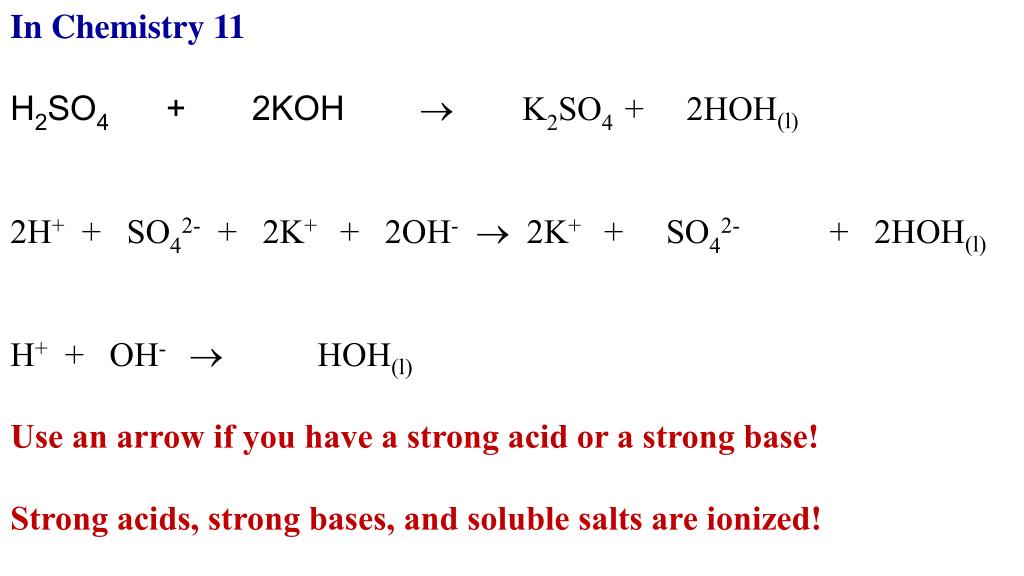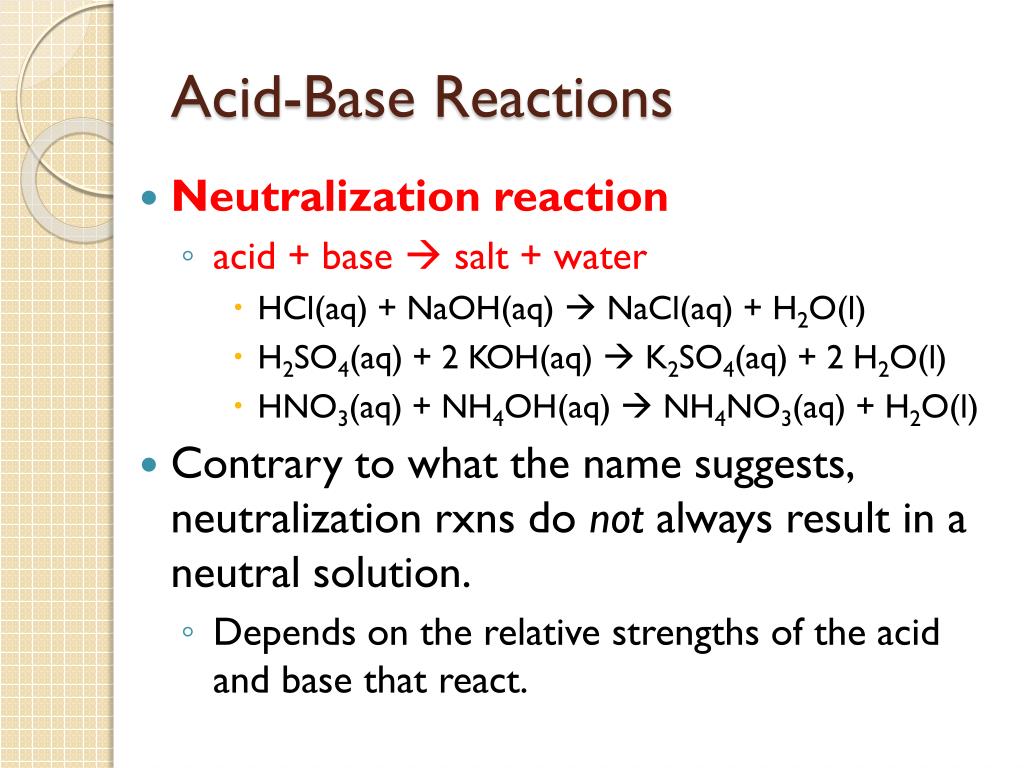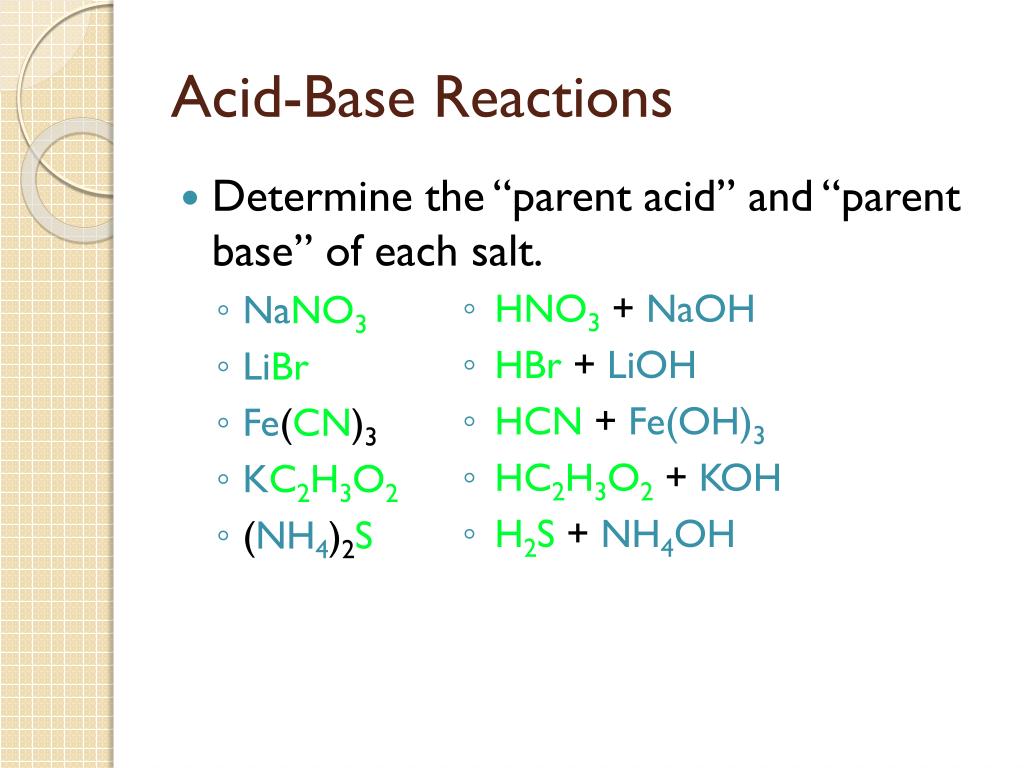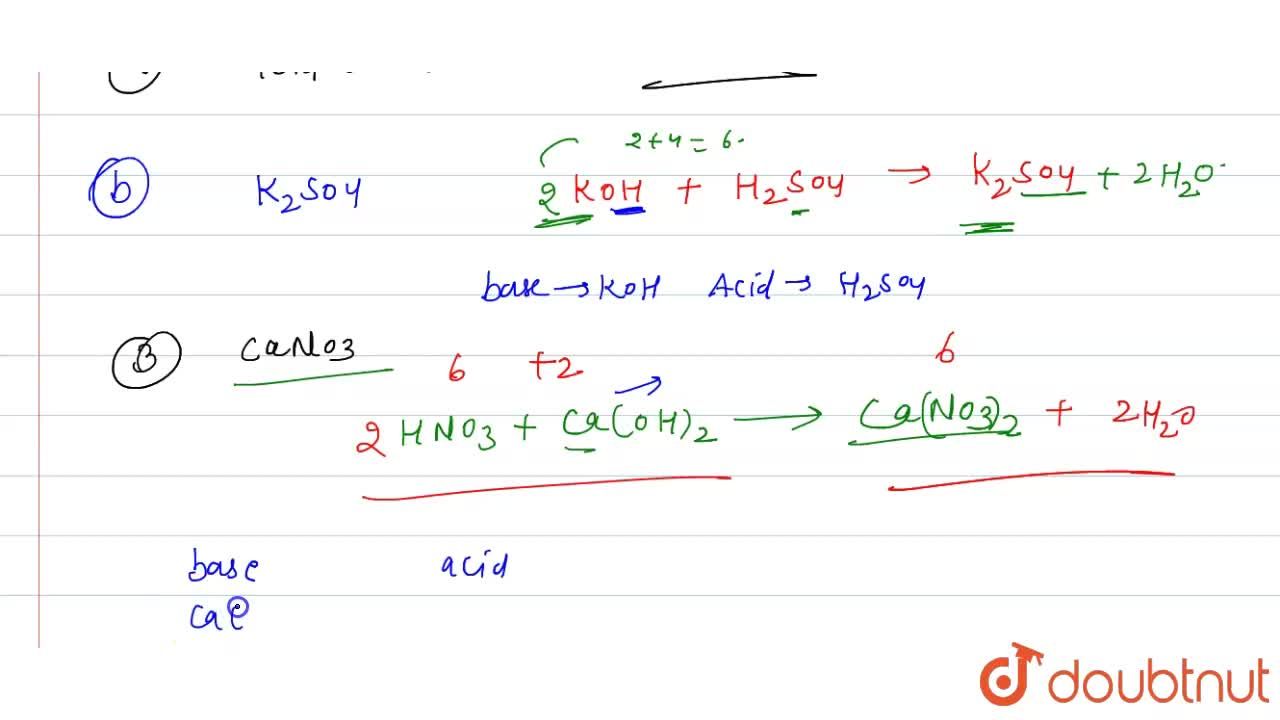
Name the acid and base from which the following salts are formed. (a) Sodium chloride (b) Potassium sulphate (c) Calcium nitrate

Classify the following salt solution in acid, base and neutral. NaCl, KNO3 , FeCl3 , CuSO4 , CH3COONa, HCOOK, CH3COONH4 , CrCl3 , K2SO4 , Na3PO4 , NH4Cl
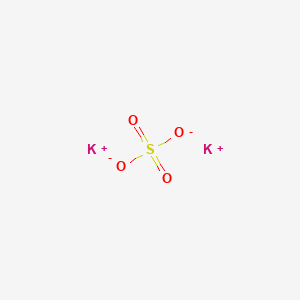
Potassium sulfate (K2SO4) - Structure, Molecular mass, Properties, Uses and FAQs of Potassium sulfate (K2SO4)