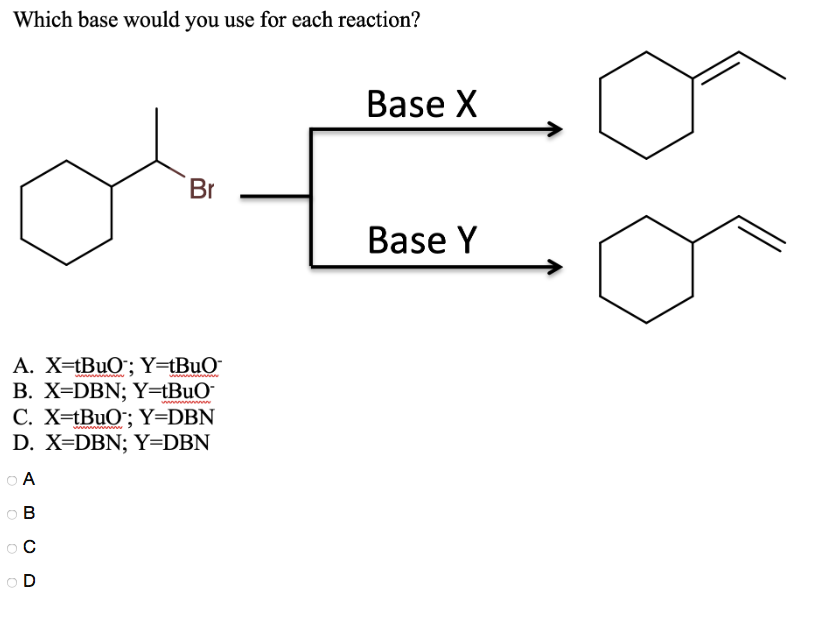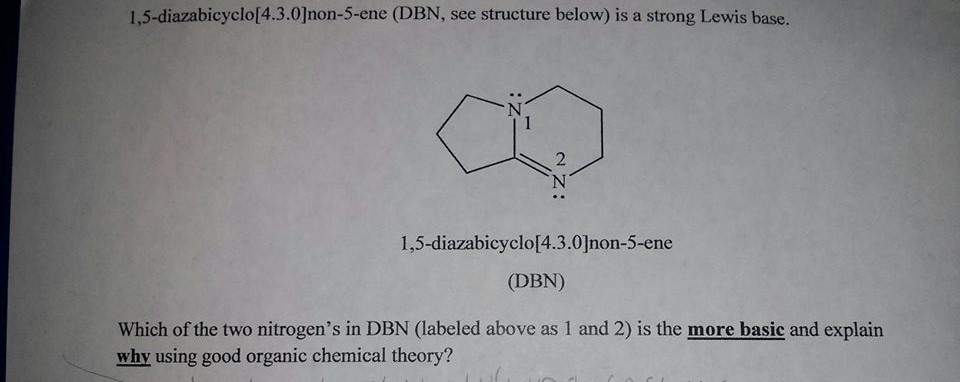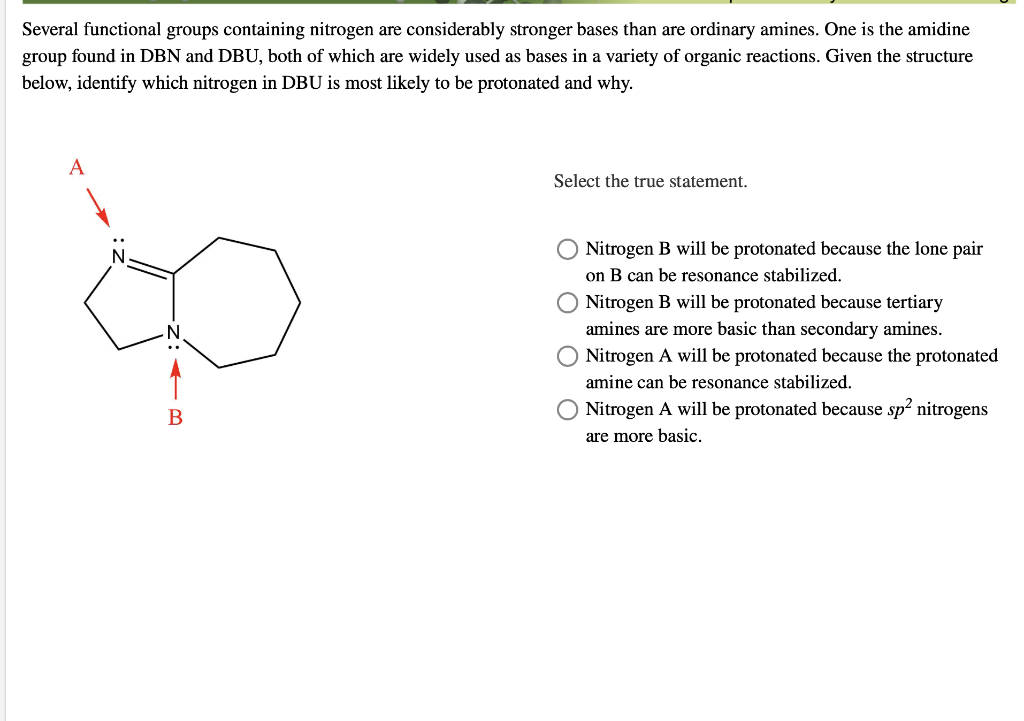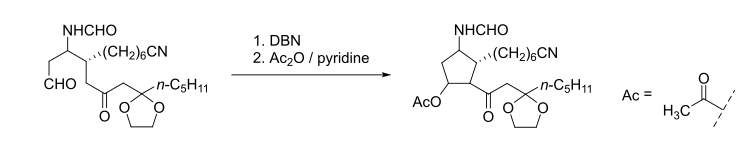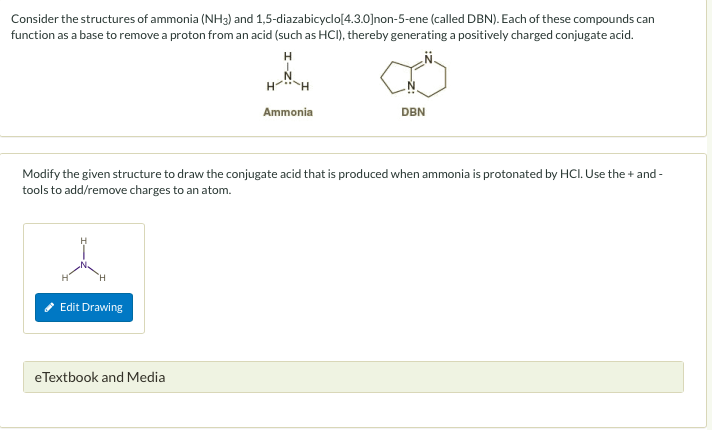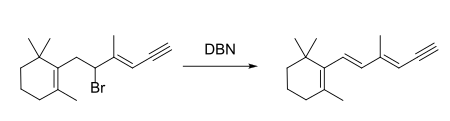
DBN is a bicyclic compound which is used as a base. What is the major product in the following reaction?\n \n \n \n \n A. \n \n \n \n \n B. \n \
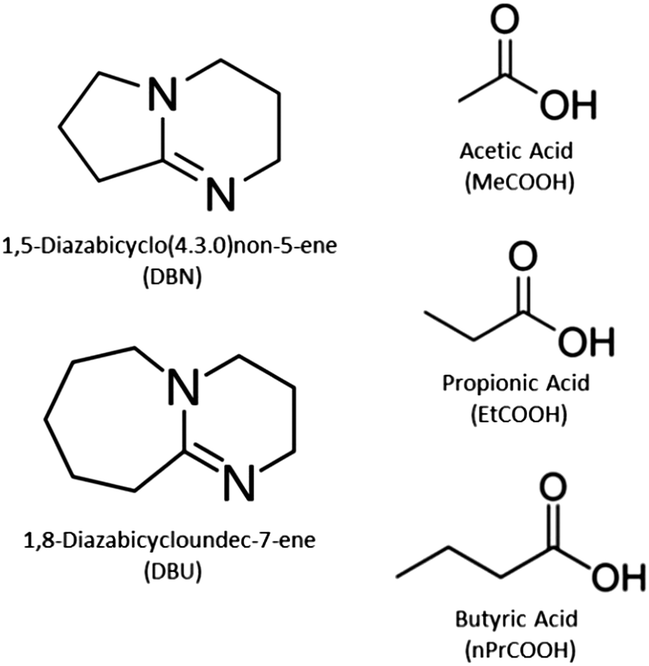
Vaporization of protic ionic liquids derived from organic superbases and short carboxylic acids - Physical Chemistry Chemical Physics (RSC Publishing) DOI:10.1039/C7CP02023F
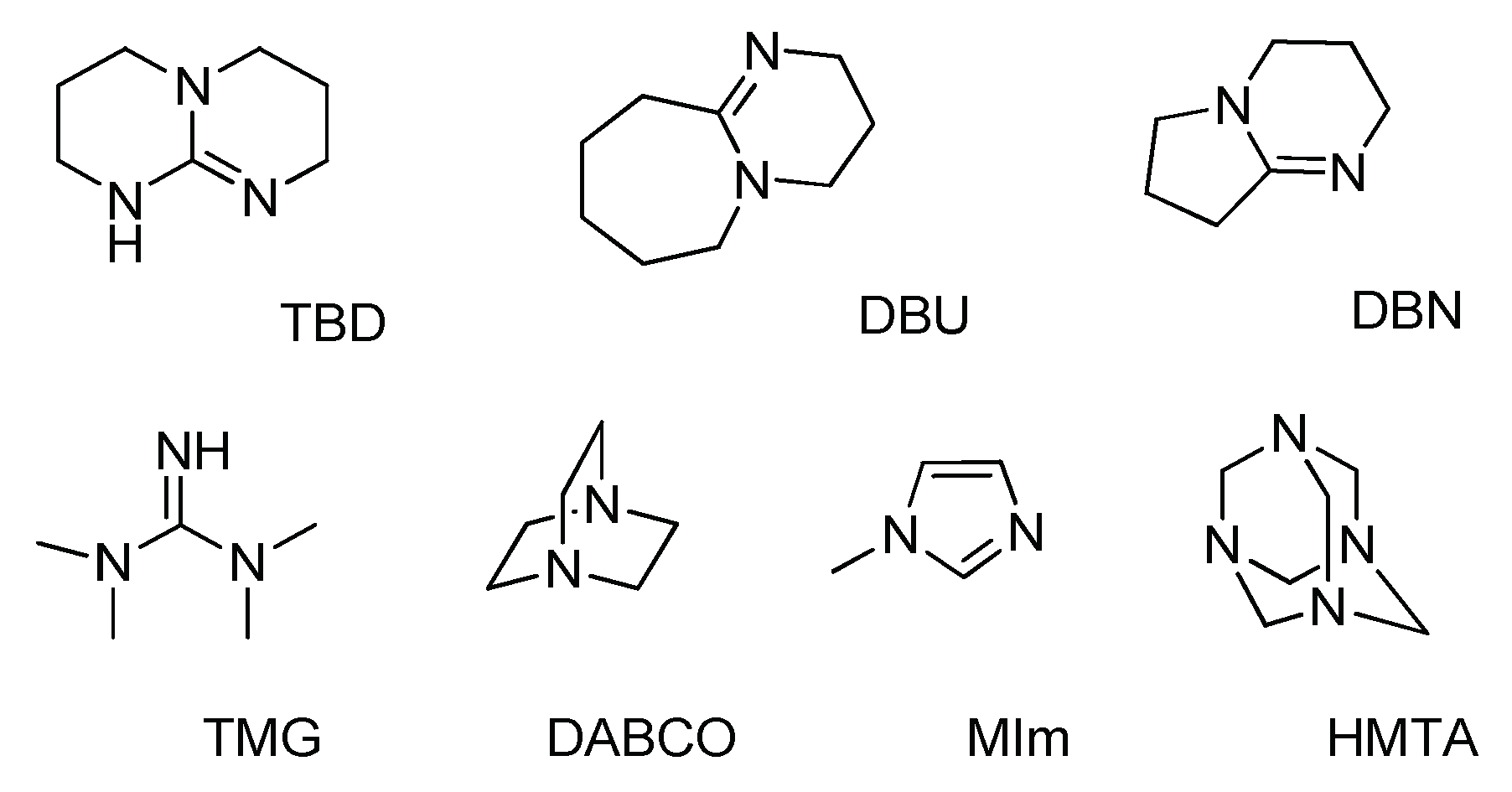
Catalysts | Free Full-Text | Organic Base-Catalyzed C–S Bond Construction from CO2: A New Route for the Synthesis of Benzothiazolones
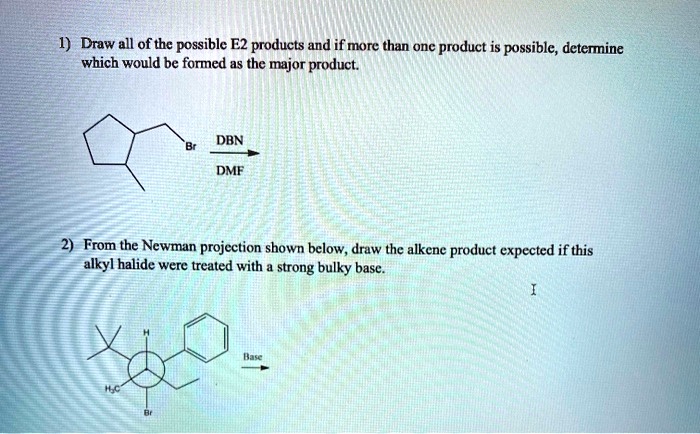
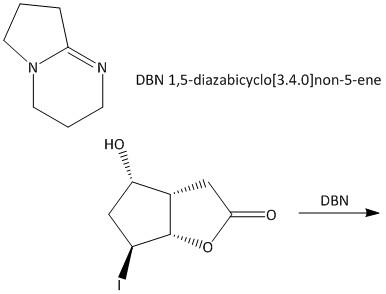
SOLVED: Draw all of the possible E2 products and if more than one product is possible; determine which would be formed as the major product: DBN DMF From the Newman projection shown

DBN is a bicyclic compound which is used as base. What is the major product in the following reaction?
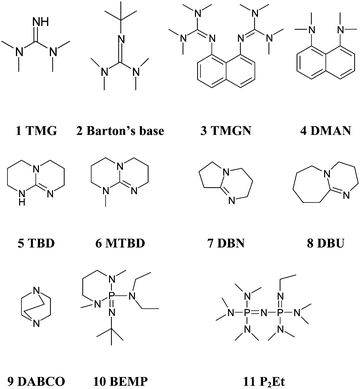
Kinetics screening of the N -alkylation of organic superbases using a continuous flow microfluidic device: basicity versus nucleophilicity - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C2OB25215E
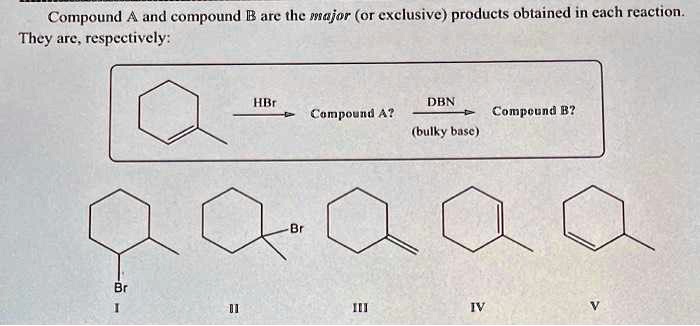
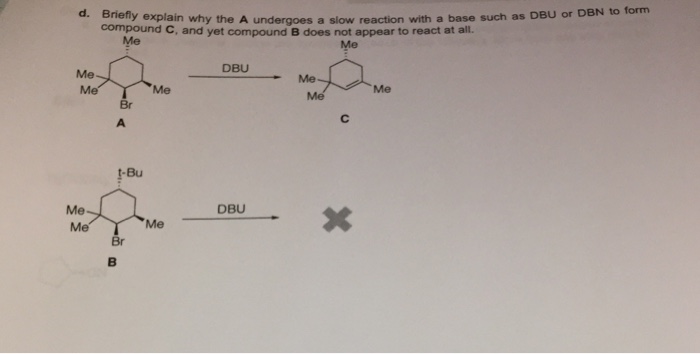
SOLVED: Compound and compound B are the wajor (Or exclusive) products obtained in cach reaction They are. respeetively: HIr DBN Campoyud A? Compcund B? (bulky busc )
Investigating the Underappreciated Hydrolytic Instability of 1,8-Diazabicyclo[5.4.0]undec-7-ene and Related Unsaturated Nitrogen