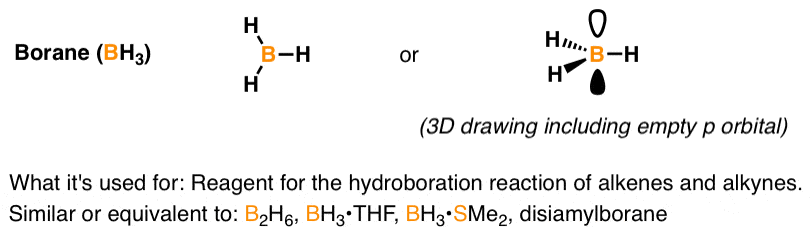
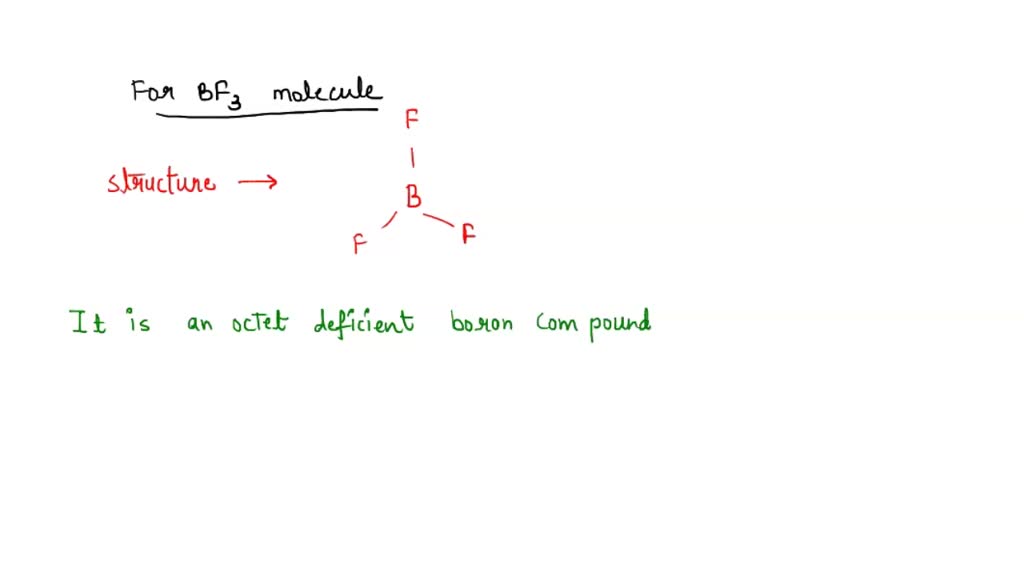
Nature and Strength of Lewis Acid/Base Interaction in Boron and Nitrogen Trihalides - Rodrigues Silva - 2020 - Chemistry – An Asian Journal - Wiley Online Library

Lewis acid-base adducts of zwitterionic alkali metal methanides and silanides with BH3 - ScienceDirect

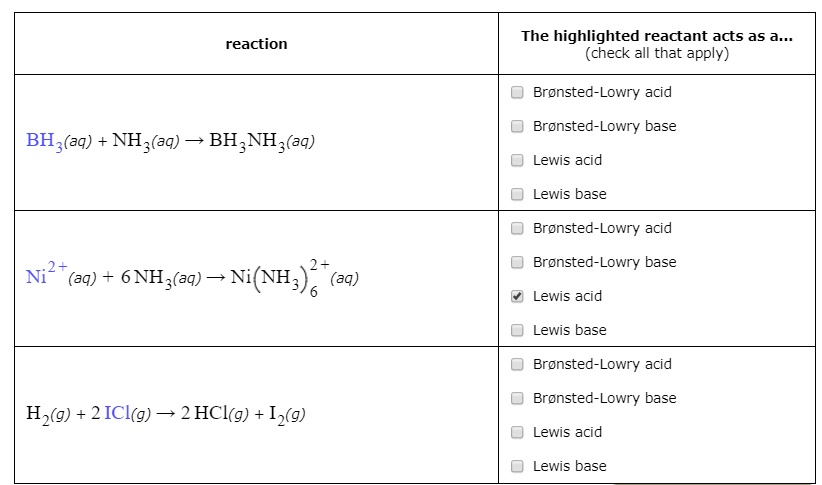
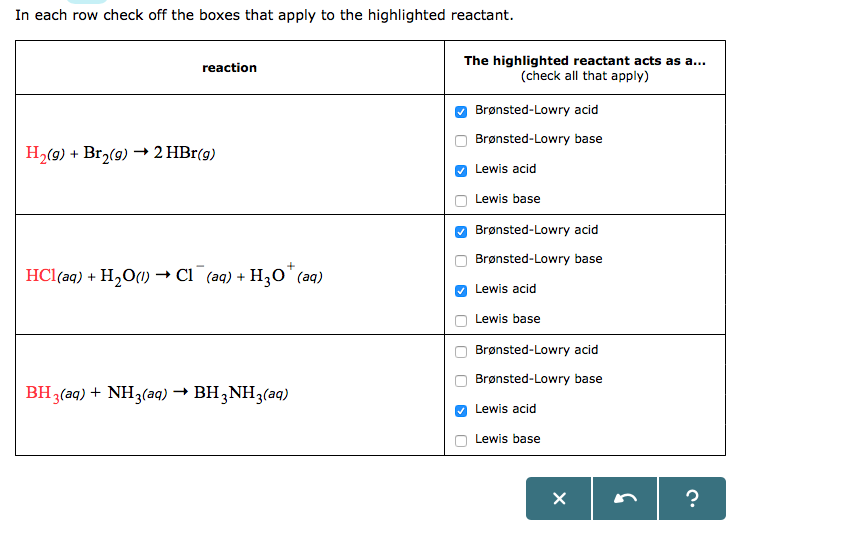
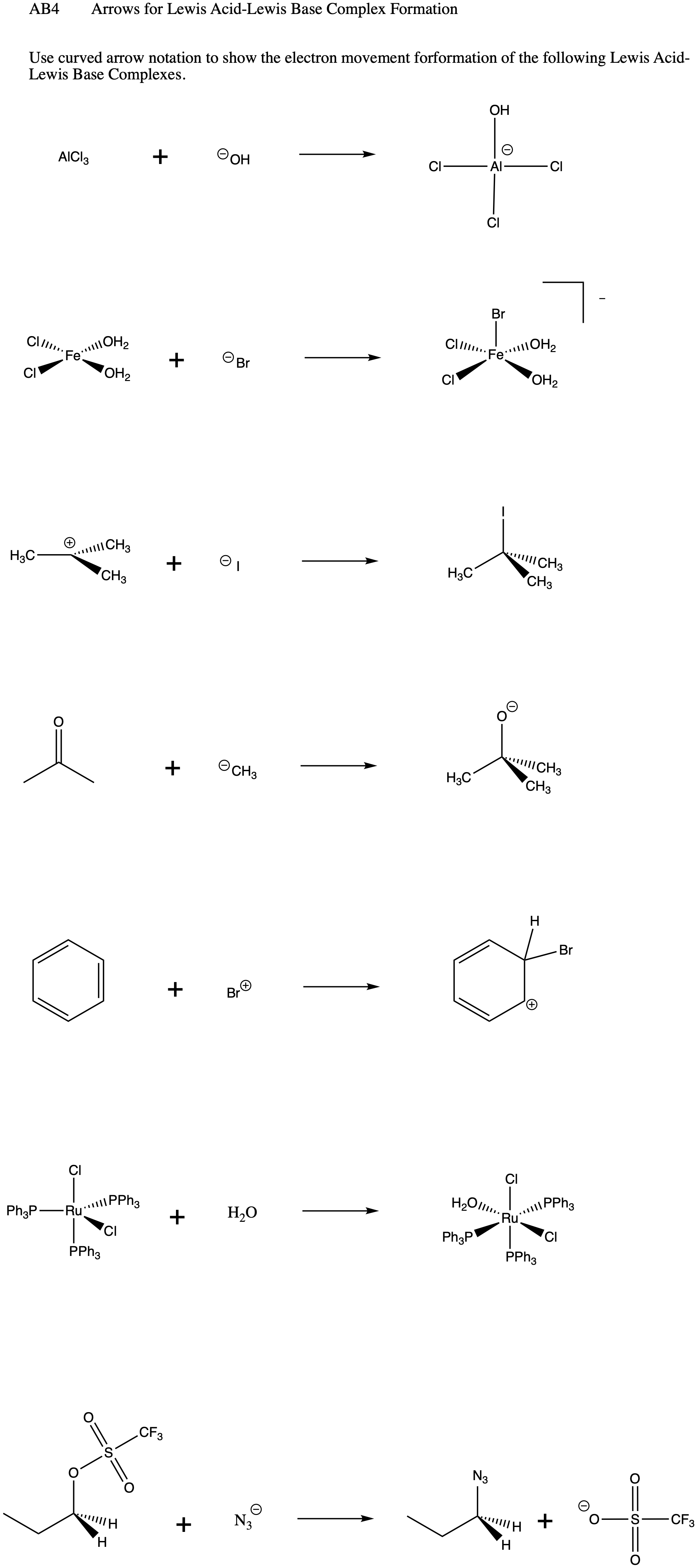
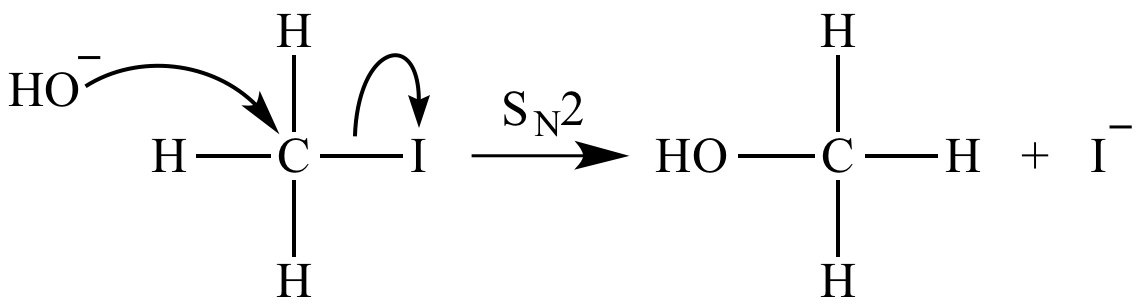
When NH3 reacts with BH3, a boron-nitrogen bond is formed joining the two compounds. Which is the electrophile in this reaction? a. Electrophile BH3 b. Nucleophile NH3 | Homework.Study.com
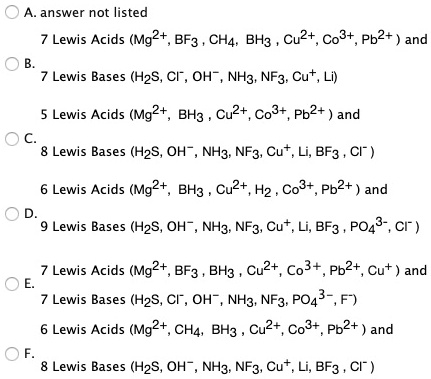
SOLVED: answer not listed Lewis Acids (Mg2+, BF? CHA; BH3 Cu2+, Co3+, Pb2+ and Lewis Bases (H2S, Cl , OH- NH3; NF3; Cut Lewis Acids (Mg2+, BH3 Cuz+,Co3+, Pb2+ and Lewis Bases (