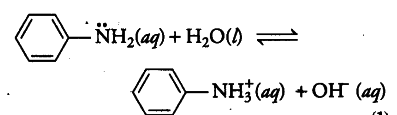
Write the equilibrium equation for the acid-base reaction - CBSE Class 12 Chemistry - Learn CBSE Forum

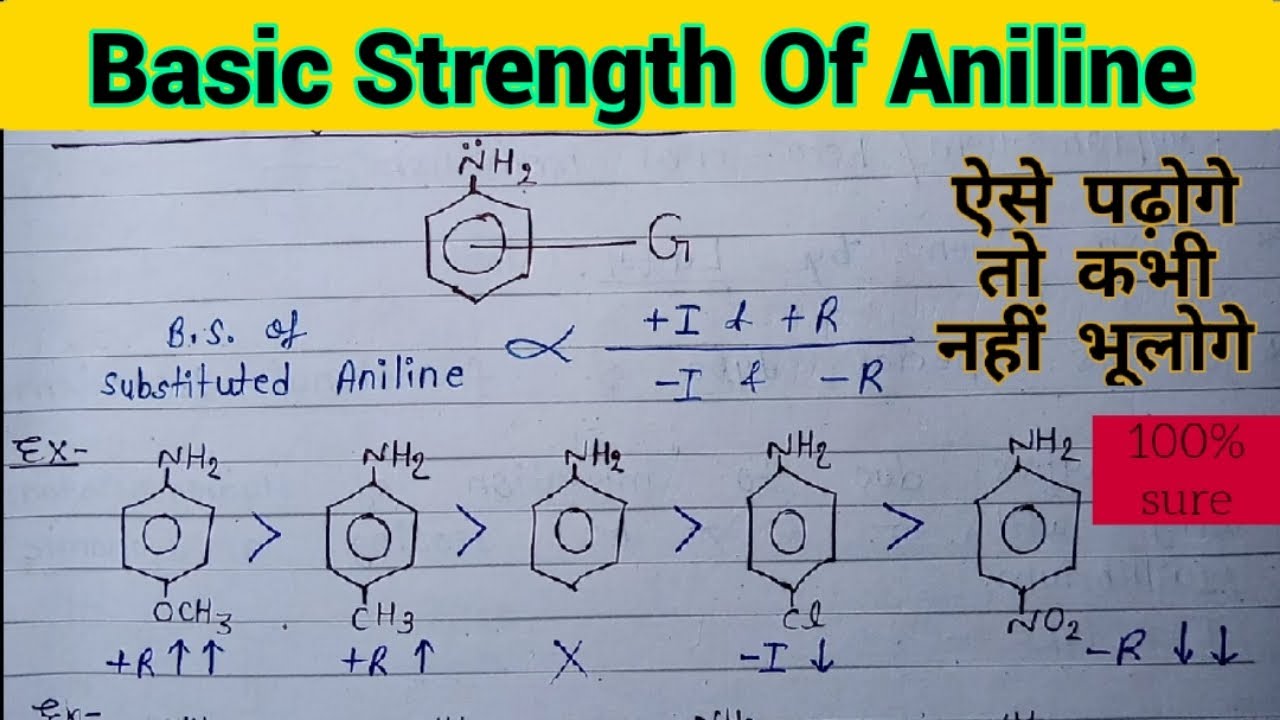
organic chemistry - Which is the strongest base among the given anilines? - Chemistry Stack Exchange
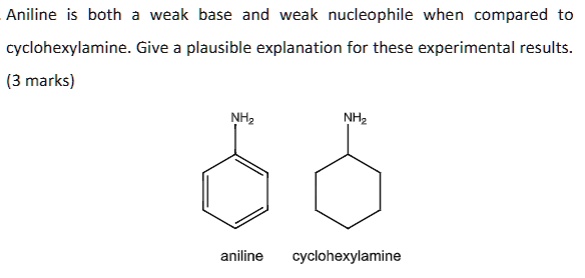
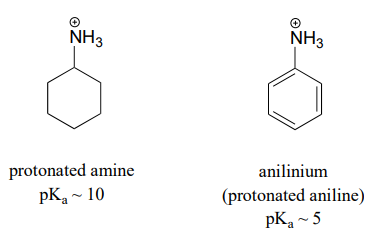
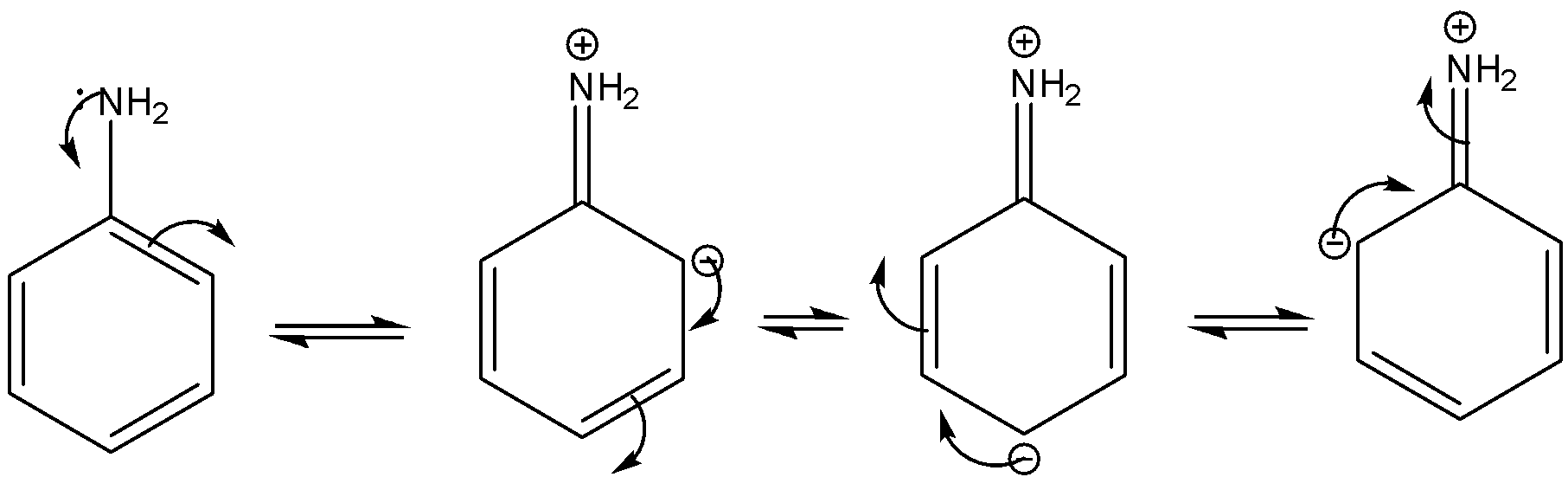
SOLVED: Aniline both weak base and weak nucleophile when compared cyclohexylamine. Give a plausible explanation for these experimental results (3 marks) NHz aniline cyclohexylamine

Propose a method to separate the following three compounds: benzoic acid, aniline and naphthalene. How would this separation look in the form of a schematic diagram? | Homework.Study.com
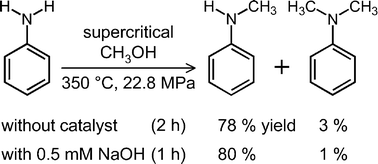
Noncatalytic mono-N-methylation of aniline in supercritical methanol: the kinetics and acid/base effect - Chemical Communications (RSC Publishing)