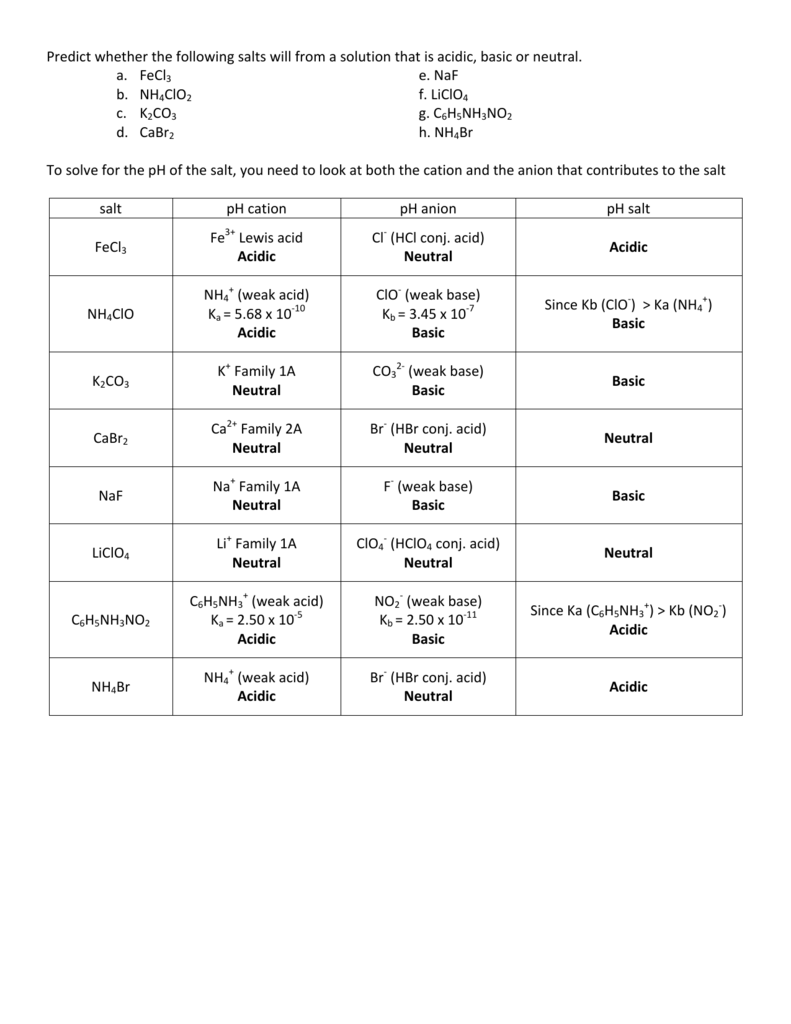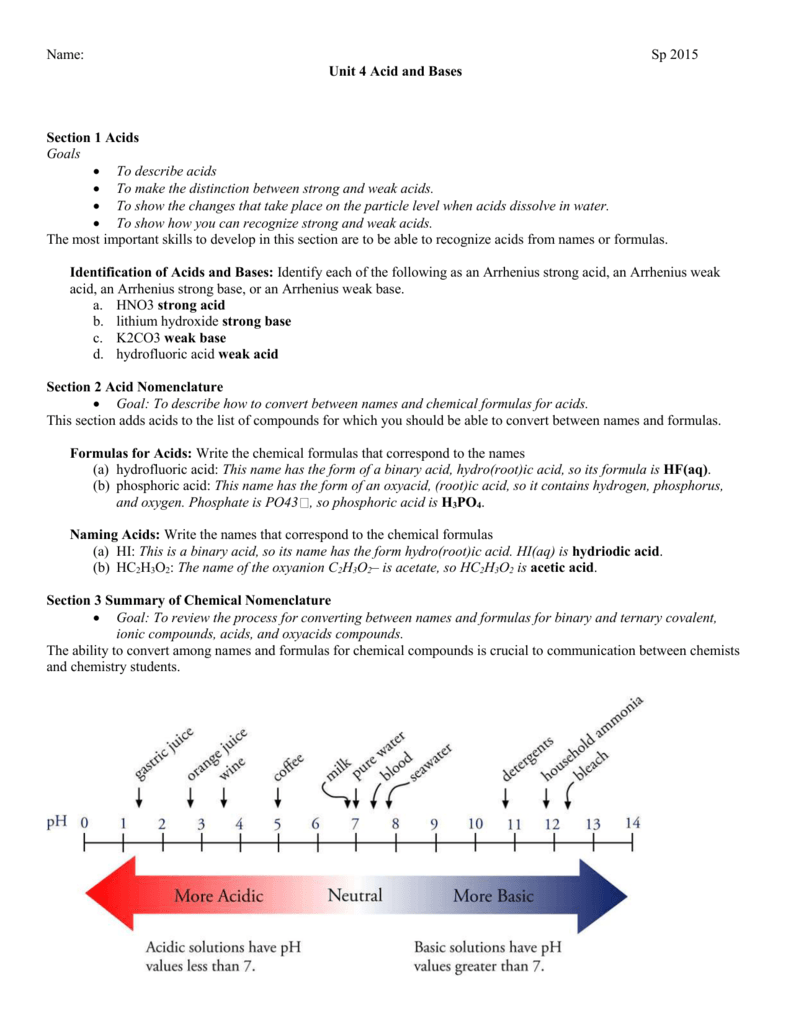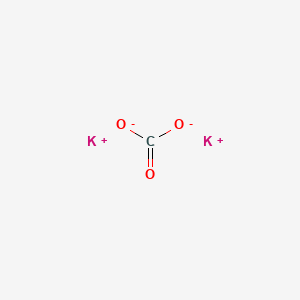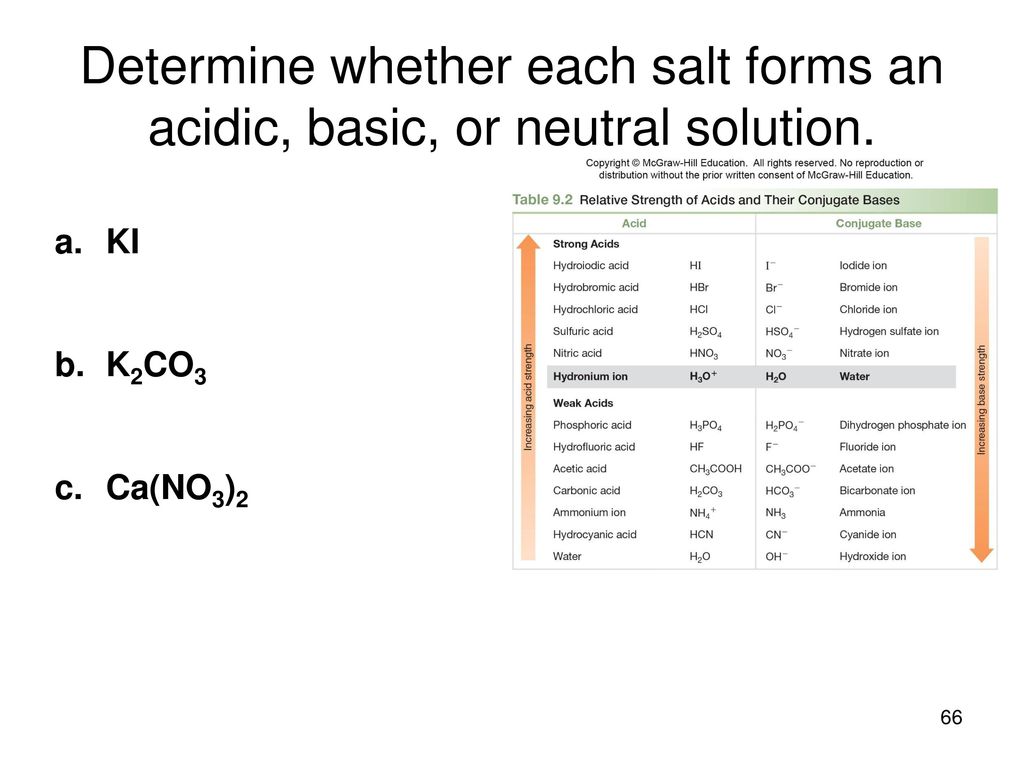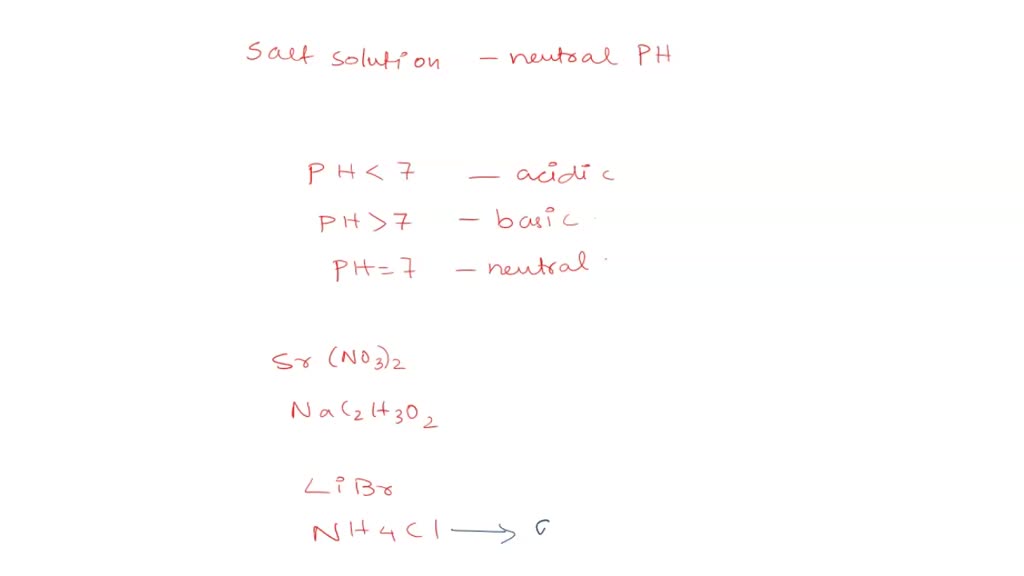
SOLVED: Select the salt from the list below which will produce a basic aqueous solution. Group of answer choices NaNO3 K2CO3 NH4Cl K2SO4
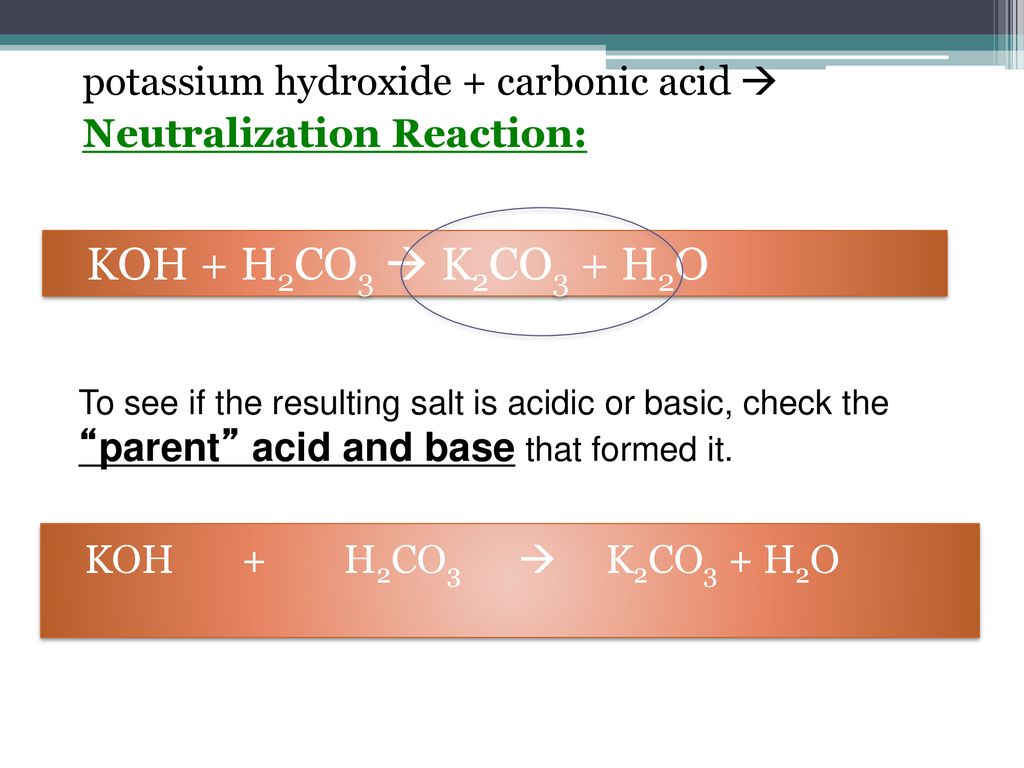
SOLVED: When K2CO3 is dissolved in water, will the solution be acidic, basic, neutral, or will more information be needed to decide?
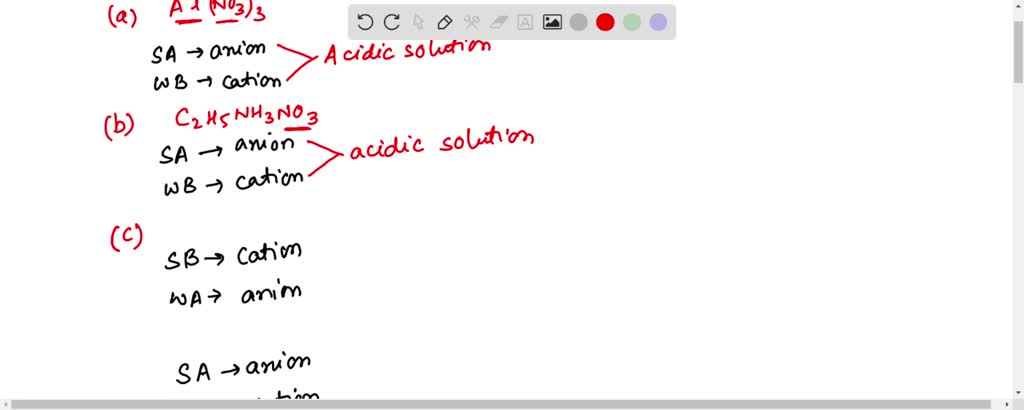
SOLVED: Determine if each salt will form a solution that is acidic, basic, or pH-neutral. a. Al(NO3)3 b. C2H5NH3NO3 c. K2CO3 d. RbI e. NH4ClO
How would you determine is the following salts will from a solution that is acidic, basic, or pH neutral? CH3NH3CN, Fe(ClO4)3, K2CO3, CH3NH3CL, RbI | Socratic